
Process monitoring vs leak detection
Sensors that detect leaks spend most of their lives exposed to little or no flammable gas. Only rarely are they needed to detect the leak, and in these moments they are useful if they can indicate the presence of a significant or unusual concentration of flammable gas. The precise concentration of the vapour cloud emanating from the leak is less important than reliable detection that such a leak has occurred.
By contrast, processes that are monitored for solvent vapour concentrations are typically enclosed. Heated spaces require an active sample drawing system. They have an intentional release of solvent at all times.
In fact, these processes are often optimised for speed and performance by safely releasing more solvent, not less; as they often operate near legal limits for combustible gas concentrations. An industrial dryer for printing, coating or laminating may be designed to operate at solvent concentrations above 25% LFL. This means the precise concentration of solvent vapours must be measured with a level of accuracy that can be difficult or impossible to attain with leak detection sensors.
Standard requirements for flammable gas detectors
Industry standards have been established which set minimum levels of performance for flammable gas detectors. In general, for a given single gas (usually methane), the sensor must be accurate under a variety of conditions to within +/-10% of the actual gas concentration. And the sensor must have a response time of less than 10 or 12 seconds. Sensors which are successfully tested and approved to these minimum standards can be listed and labelled, for example ‘FM Approved’.
Special requirements for solvent vapour monitoring
Solvent vapour monitoring in ovens and dryers has special requirements, which are set out in the NFPA-86 code.
Experience has shown that explosions in ovens and dryers can happen very quickly. The code explains that an abnormally high solvent concentration should be detected and corrected within five seconds or less. Some approved sensors, having response times up to 12 seconds, clearly are not suitable. Other sensors which appear to be fast enough are in fact slower than expected: Diffusion sensors adapted for use with flow collars can be twice as slow for solvents, which diffuse more slowly, than they are for methane, the gas for which their speed was determined. So a response time of five seconds for methane might translate into a response time of 10 seconds for a solvent. Sampling systems should be optimised for speed, with short tubing lengths, high flow rates and very low filter volumes.
During an accident, speed and accuracy are identical. When the solvent concentration is increasing at +10% LFL per second, a sensor that lags behind by five seconds displays a reading 5s x 10 % LFL/s = 50% LFL below the actual concentration in the dryer. A 50% LFL alarm from such a sensor occurs when the dryer is already at 100% LFL.
Most dryers are used for more than one solvent. Accuracy depends completely on how the sensor responds to each solvent.
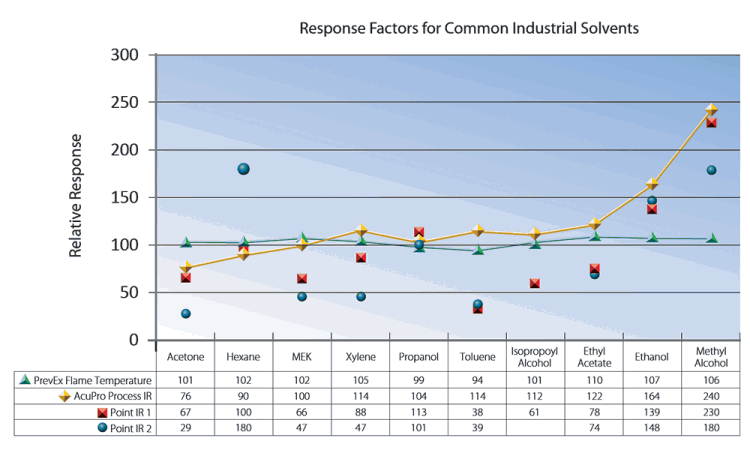
The measuring principle of the sensor is important. Typically, catalytic bead response factors are within +/-35%, flame temperature within +/- 10%, and infrared can easily vary from -50% to +150% or more; however, some process infrared sensors with multiple detectors can provide more precise readings. The code requires calibration for the solvent to which the sensor is least sensitive.
If the response to one solvent is several times lower than another, as for point infrared, the reading can be several times higher than the actual concentration, making it impossible to operate the dryer at its intended speed, due to false alarms.
To find how a sensor responds to different solvents, compare that sensor’s response factors. Check the effect the response factors have on normal operation, and on the alarms. Select the solvents of interest. ‘Calibrate’ for the lowest response factor, as required by code. Check the accuracy for the other solvents.
The more solvents, the more chance for error. The Point IR 1 response factors in the table vary from 38 up to 230. This means the indicated reading can be up to 230/38 = six times higher than the actual solvent concentration. A dryer that is to handle all those solvents would have to operate below 50/6, or less than 8% LFL, to prevent a false alarm. Whereas the PrevEx Flame Temperature varies from 94 up to 110, which means the indicated reading can be up to 110/94 = .17 higher than the actual solvent concentration.
Calculating a composite response factor, or ignoring ‘minor constituent’ is not allowed unless the amount of error could be small (a few percent at most). Only the method of calibration for the least sensitive solvent is recognised in the code.
Questions to consider:
1. Is the sensor approved by Factory Mutual? Does the approval cover the entire device, including the sampling system, and not just the sensor element?
2. Does this approval include the response to solvents, not just methane or propane?
3. How quickly can the device make an alarm? Is the time-to-alarm less than a few seconds?
4. Does this ‘time-to-alarm’ include the ‘sample transport time’, that is, the time to extract a sample and deliver it to the sensor, and to filter it for particles and contaminants? If not, how much more time is needed?
5. Is the specified response time true for solvents, not just methane?
6. For the solvents to be measured, now or in the future, how different is the response of one to another? How much error results from measuring different solvents?
7. Is it possible to easily mis-calibrate the sensor, so that it fails to give an alarm even though a flammable concentration is present?
8. Can the device be calibrated according to code and still allow dryer operation, without false alarms?
9. Is the sensor and sampling system heated to a temperature above the flash point(s) of all solvents that are used, or might be used?
10. Is the entire system heated to a temperature above the dew point, especially of water vapour? Can water vapour interfere with the operation of the device?
11. Does the device remain accurate under the temperature or pressure changes that are expected?
12. Is there a means to test and verify proper operation using a test gas? Is the test automated so it can be performed on a monthly basis, as required by code?
13. Is the detection system considered an analyser (for process) or a sensor (for leak detection)?
| Tel: | +27 11 918 6994 |
| Email: | [email protected] |
| www: | www.e-analytics.co.za |
| Articles: | More information and articles about Elemental Analytics |

© Technews Publishing (Pty) Ltd | All Rights Reserved