

Insurer Aon Corporation reports1 that the average total insurance limit purchased by food system, agribusiness and beverage (FAB) companies to cover product recall/contamination is a cool R180m (US$25 million).
Recall risk
The same report lists product coding, smaller lot sizes, as well as forward and backward traceability as factors affecting the scope and size of recall and the FAB business’s vulnerability to contamination.
Two kinds of traceability
As I wrote in an earlier article2, there are two principal kinds of traceability to be addressed in meeting current ‘farm to fork’ regulatory requirements:
* Chain traceability.
* Internal traceability.
Chain traceability focuses on inter-organisational transactions – for instance between shipper and manufacturer – and typically requires data capture relating to material movements at a manufacturer’s material receiving and dispatch bays.
Internal traceability is about intra-organisational transactions – for instance the dispensing and addition of materials for batches of semi-manufactured and finished materials – and typically requires data capture at weighing and processing operations within the manufacturing complex.
This article concentrates on internal traceability in manufacturing, with a significant emphasis on batch manufacturing and formulation management.
Internal traceability
The successful implementation of internal traceability requires that the following be addressed:
* Materials receiving and marking.
* Change management.
* Control Recipe interface.
* Control Recipe material consumption.
* ERP consumption interface.
* Material dispatch and marking.
* Targeted recall.
Materials receiving and marking
Within the manufacturing organisation, the materials receiving function forms the interface between chain and internal traceability systems. Data capture required to meet chain traceability quality metrics informs internal traceability systems about the presence and availability of materials, their internal lot numbers and physical characteristics like specific gravity (SG), potency and expiry date.
Because each manufacturer may have its own receiving/Q A release system – which may be part of their accounting or business system – it is beneficial to create a data transfer interface at this point between the receiving/Q A release system and the batch management system. This automates the data exchange between the two systems improving efficiencies and eliminating transcription errors. Figure 1 shows the GUI for an interface that imports inventory received data from an external system into BatchManager.
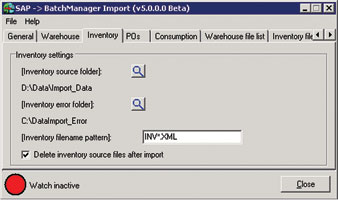
The standard operating procedure (SOP) for material should call for materials to be clearly marked with the organisation’s material code, material description, expiry date and unique internal lot number. Our experience at ProLoCon has shown that there is great value in including item code and internal lot number in machine-readable form as well as human-readable form. This facilitates subsequent data capture using RF readers or barcode scanners when materials are moved within the organisation – for instance from receiving to quarantine or from quarantine to general warehouse/line-side warehouse – at points of material consumption within the manufacturing process. Concatenating material code and internal lot number into a single machine-readable code allows both fields to be read at a single scan. (See Figure 2).

Change management
Change management is an aspect that is often overlooked when planning traceability systems. And yet changes to physical characteristics of materials may significantly influence semi-manufactured and finished goods. For instance, if a user changes the SG of a liquid component, or the potency in an item master record then this will impact on the quantity of that material to be dispensed as part of the Control Recipe. (See Figure 3).
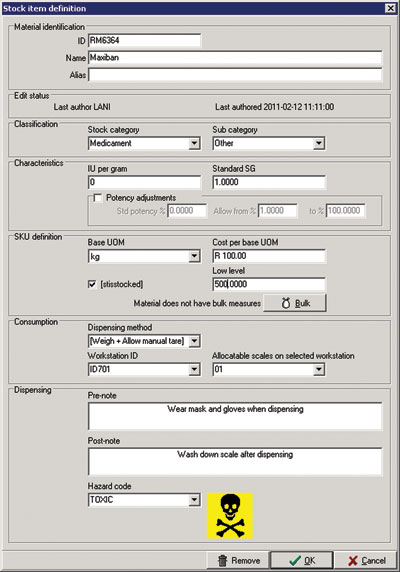
If changes to material characteristics are made in a system that is not responsible for execution of Control Recipes (the batch management system) then there needs to be an interface between the external system and the batch management system to synchronise such changes, and the date and time of these changes need to be recorded against a duly authorised user.
Where Site Recipe management is handled within the batch management system (as opposed to within an ERP system like SAP) changes to the Site Recipe bill of materials (BOM) should also be time-stamped and the change associated with a known and appropriately authorised user.
Control Recipe interface
If the system that is responsible for site recipe management is not the same system that is responsible for batch execution (for instance site recipes may be managed in a system like SAP or SYSPRO) then the ideal is for the scheduling event in the host system to trigger a transfer of data to the batch management system. Automating this interface eliminates transcription errors and ensures that the scheduling status between the two systems is near synchronous.
Control Recipe material consumption
The accurate recording of materials (quantities, lot numbers and other key data) incorporated into semi-manufactured and finished goods is the core requirement for a successful internal traceability system.
The way in which the dispensing (weighing) is done varies from industry to industry and is also influenced by the target weight. Most international regulatory bodies for the pharmaceutical industry require that materials are manually weighed by suitably qualified dispensary personnel and then the weight checked and signed off by a qualified manufacturing pharmacist. In other, slightly less stringent, environments like the manufacture of foodstuff, nutriceuticals and personal hygiene it may be legitimate to automate weighing, but often there are high value low quantity additions that need to be manually dispensed because the cost of automating the accurate addition of 1 g of a flavour or colouring may be prohibitive.
To accommodate these diverse requirements the batch management system should be capable of interfacing directly to scales and/or industrial weighing terminals to support manual weighing and should also be able to provide the necessary data to external scada and plc systems. And because the batch management system may the short- or long-term repository for traceability data it needs to be capable of receiving back consumption data (quantities, lot numbers and other key data) from those external systems.
When the batch management system interfaces with scada and plc systems such systems are often drawing materials from silos and have no knowledge of the actual lot number of material that they are weighing off. There are two ways of handling this dilemma:
* Empty the silo.
* Assume linear flow.
In either case the date and time of each transfer of material into the silo needs to be recorded.
For indisputable traceability no differing lots of material should be mixed within a silo and each silo should be emptied before recharging with a different lot of material. The reality is that emptying silos before recharging may not be practical or may prove to introduce unacceptable manufacturing inefficiencies.
If each recharge of the silo is recorded within the batch management system with a timestamp and quantity and linear flow is an acceptable assumption then by treating the silo as a FIFO bin the batch management system can incorporate algorithms to allocate the appropriate lot(s) of material to the BOM line of the correct Control Recipe.
ERP consumption interface
If overall plant inventory management and/or long-term traceability consumption data retention is not the responsibility of the batch management system then an automated interface between the batch management system and the system(s) responsible for these aspects keeps inventory data synchronous and accurate across systems.
Material dispatch and marking
Experience has shown that the batch management database is an ideal source of data for detailed finished goods labels since all the header data (product code, product name, usage notes, batch number, expiry date...) and detail data (typically ingredients) are available in that system. Figure 4 shows a label generated using data from a batch management database.
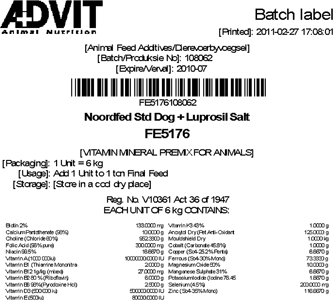
When designing an overall traceability system, readers should remember that the incorporation of material into semi-manufactured and finished product (consumption) is not the only possible destination of an incoming material: it may be returned to the supplier, sold on in its raw state (as received with no manufacturing) or it may be scrapped. These aspects are best dealt with as part of the chain traceability system.
Final dispatch of materials for shipment falls back in the realm of chain traceability.
Targeted recall
One of the primary regulatory rationales for traceability systems is to facilitate a rapid response throughout the supply chain to contamination or out-of-specification events. From an internal traceability perspective this means that the system in which traceability information is stored needs to be queryable to support both forward traceability and backward traceability.
About the author

Andrew Ashton has electrical, mechanical and business qualifications and has been active in automation and process control since the early 1980s. Since 1991 he has headed up a company that has developed formulation management systems for the food, pharmaceutical and chemical manufacturing industries and manufacturing solutions involving the integration of various communication technologies and databases. Developed systems address issues around traceability, systems integration, manufacturing efficiency and effectiveness. Andrew is a contributing editor for S A Instrumentation and Control.
References and resources
1. Aon Corporation, 2010 U.S. Industry Report: Food System, Agribusiness and Beverage, http://tinyurl.com/63cbywj.
2. SA Instrumentation and Control, Traceability: Alpha and Omega, http://tinyurl.com/6e3zzm3.
3. European Commission, Food Traceability factsheet, http://tinyurl.com/2nyddz
| Tel: | +27 11 543 5800 |
| Email: | [email protected] |
| www: | www.technews.co.za |
| Articles: | More information and articles about Technews Publishing (SA Instrumentation & Control) |

© Technews Publishing (Pty) Ltd | All Rights Reserved