

In the food, feeds and pharmaceutical industries, regulatory compliance means that traceability and weighing are inseparable.
Introduction
Batch weighing activities are an important part of many manufacturing processes – especially in the food, pharmaceutical and animal feed industries. One of the problems that end users face is that their batching systems are not well integrated with their overall data management. This causes inefficiencies and results in chemical engineers, nutritionists, materials managers and manufacturing staff spending time performing critical tasks in unmanaged spreadsheets and paper-based production reporting.
This results in systems which do not comply with the ever-tightening regulatory and GMP requirements of many industries.
A new direction
Advit Animal Nutrition (Advit), a manufacturer of vitamin and mineral supplements to the animal feed industry, based in Sebenza, South Africa, has recently commissioned a system that addresses many of these issues, by integrating recipe handling data from quotation.
User requirements
Advit was looking for a solution that would replace its unmaintainable MSAccess-based custom solution and standalone BarTender labelling system with something that would be able to provide its animal nutritionists with a tool to:
* Develop and maintain master recipes in a controlled way.
* Link to product registration number (in terms of the Fertilizers, Farm Feeds, Agricultural Remedies and Stock Remedies Act, 1947 (Act No. 36 of 1947).
* Support the concept of material potencies.
* Support working in IU for vitamins (see sidebar below 'What is an IU?').
* Calculate costs and provide formatted quotation data.
For overall manufacturing operations Advit wanted a system that would:
* Provide data continuity and integrity from quotation stage to packed goods ingredient labelling.
* Meet the local and international requirements for traceability and recall.
* Link to Mettler ID7 and IND690 terminals for manual weighing.
* Link to Wonderware InTouch for automated weighing.
* Eliminate the need for Wonderware InBatch.
The Advit system architecture is shown in Figure 1.
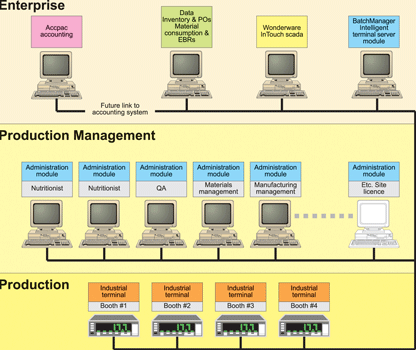
BatchManager baseline extended
ProLoCon has been working on further developing the intelligence of objects in its BatchManager application to suit the various business rules that are important to its clients in the regulated industries. These business rules relate to the verification of inventory availability, inventory expiry, the requirement to consume raw materials in order of expiry date (stock rotation), and to many more detailed aspects of manufacturing dispensing.
Historically BatchManager, like many of its peers, has focused on weighing (dispensing) operations and the associated inventory management activities like stock reporting and stock consumption related to dispensed materials. For raw material, semi-finished material and finished goods traceability pre- and post-batching ProLoCon developed its materials transfer module (MTM) and bar code module (BCM).
Master recipes and quotations
To meet Advit’s needs, ProLoCon enhanced the BatchManager baseline code that handles master recipes so that once a recipe version is authorised it can no longer be edited. This means that each change to a master recipe results in a new controlled version of that recipe, thus providing full reverse traceability from any instance of a control recipe to the correct instance of its master recipe. At the same time, the code was modified to support cost calculations and the concepts of inactive, active, superseded and retired recipe versions.
These changes allow the animal nutritionists to develop formulations for quotations in a version controlled way that ensures that production batches cannot be scheduled from such unauthorised or inactive recipes.
The support for IU and potencies means that the nutritionists can specify eg, 1 000 000 IU of Vitamin C and the software can then calculate the gram equivalent of that. Or they can specify that they require 10 g of Vitamin B2 and the system will calculate that that is equivalent to 12,5 g of Vitamin B2 80% (Riboflavin).
Master recipes can be assigned to a single client (as is often the case) and can also be assigned to a specific processing line.
One of the outcomes of these changes has been the ability to provide more comprehensive master recipe reporting:
* Formulation detail report (an internal report).
* Formulation quote report (a detailed nutritional report for the client).
* Internal price list report (prices of all master formulae in a user-selected range).
* Client price list report (prices for all master formulae for a particular client).
The typical flow of nutritionist activities are shown in Figure 2.
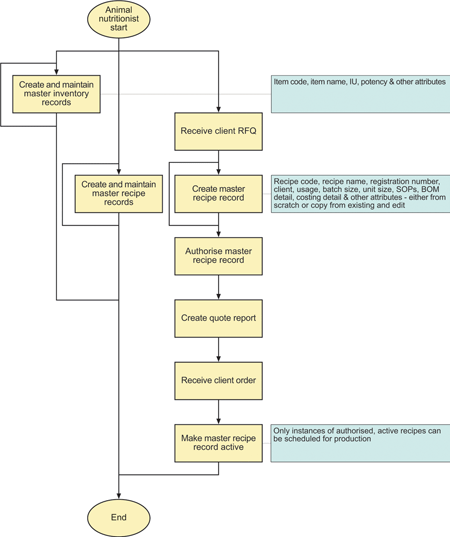
Inventory
Minor changes were made to the BatchManager baseline table structures to accommodate data relating to IU, unit costs and categorisation of item codes.
Comprehensive reporting allows the materials manager to determine inventory requirements based on production schedule and inventory re-order levels.
Typical materials management tasks are outlined in Figure 3. ProLoCon’s MTM is the front-end for BatchManager. It records materials receipts and creates a GRN, associating each receipt with the purchaser order number, supplier, supplier COA, supplier lot number, expiry date and other necessary traceability information. The receiving event also generates a unique internal lot number and uses the BCM to create a receiving label, which encodes the concatenation of item code and internal lot number in a bar code. This means that when the material is dispensed it can be identified in an error-free way from a single bar code scan.
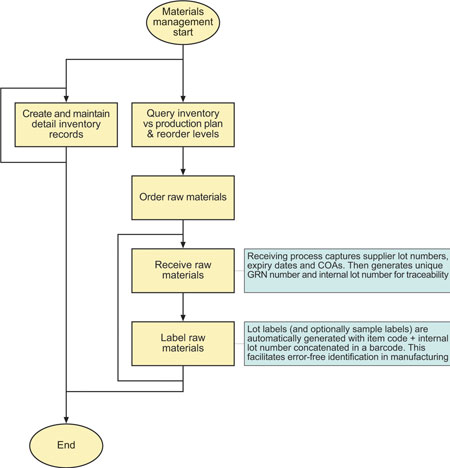
Manufacturing management
Please refer Figure 4. When the first client order for a quoted master recipe is received, the responsible nutritionist makes that master recipe active, which then makes it possible for manufacturing management to schedule batches based on it.
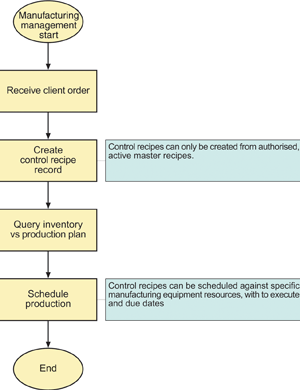
In the case of Advit, master recipe batch sizes are typically 'units' of 1 to 35 kg. When manufacturing management schedule a batch they specify the actual batch size. BatchManager incorporates a calculator that simplifies this up scaling so that the manufactured batch can be scheduled as an integer number of 'units'. They can also specify the production line on which to manufacture the batch. Depending on the configured business rules, the application allocates a sequential (internal) shop order (manufacturing requisition) reference number and sequential (external) batch number for this production order.
The manufacturing management team also enter (or accept default values) for the planned start date, due date and (rule-based) expiry date. To provide manufacturing flexibility production orders can also be prioritised.
A large number of preconfigured reports (see Figure 5) are available and where these do not fit the bill, a SQL-based report macro language allows users to extend the reporting.
Manufacturing execution
At Advit, there are both manual and automatic weighing stations. This is quite common in food, pharmaceutical and chemical applications, where either for regulatory compliance reasons or precision reasons it is not possible to fully automate weighing.
On this project, in all cases the source of the quantity targets and tolerances for the dispensing (weighing) and the destination for the weighing archives is the BatchManager database. In other projects the source of control recipes and the destination of weighing archives may be a client’s main business system (BPCS, ProdStar, SAP, SYSPRO …).
Weighing architecture
There are four manual weigh stations, each comprising:
* Mettler ID7 or IND690 industrial terminal.
* 30 kg
* 1g scale.
* Bar code scanner.
There are two automatic scales controlled by a Siemens PLC through a Wonderware InTouch scada. One scale is fed by two bulk silos and the second fed from six smaller silos. These scales feed the blenders into which the manual components are hand-added.
Currently there is no automated check of these manual additions. It is envisaged that in future ProLoCon’s Check Point Module will be implemented for this.
Weighing operation
Whether the weighing is taking place at one of the industrial terminals or via the scada, the operation is very similar (Refer Figure 6).
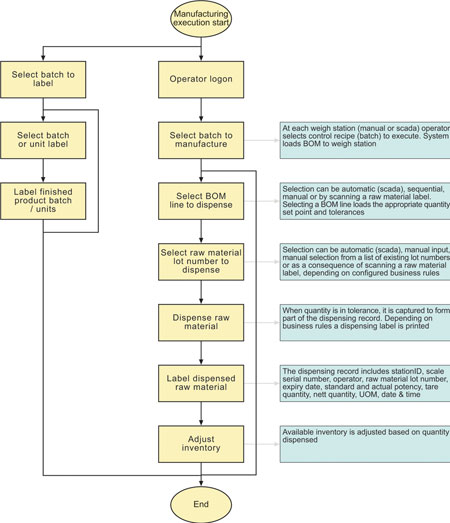
The operator logs on to the weighing interface (ensuring traceability to a user and weighing station) and then selects a job to weigh. The job list comes from a SQL query on the BatchManager database.
Once a job has been selected the weigh station allows the user (or automation system) to obtain the detail of each BOM line and to weigh against that, as long as a valid raw material and lot number is supplied. On manual terminals, BatchManager will not allow overweighing of materials. On automatic systems that task is left to the automation system.
Batches are only considered complete by the system when all ingredient (BOM) lines have been dispensed and the quantities are within tolerance.
As each ingredient weighing is performed, the available inventory is reduced based on the specific lot number dispensed.
To provide full traceability, the dispensing record links back to the job BOM and also includes the following fields:
* Stock name.
* Stock ID.
* User name.
* Station ID.
* Log time.
* Tare quantity.
* Nett quantity.
* Unit of measure.
* Lot number of ingredient.
* Expiry date of ingredient.
* Scale ID.
* Potency of ingredient lot.
* Standard potency of ingredient.
* SG.
Finished goods labelling
For vitamin and mineral feed supplements the finished product has to be labelled with batch number, product name and code, registration number (in terms of Act No. 36 of 1947), expiry date and ingredient information. Since all of this information resides in the BatchManager database a labelling application was developed which prints the necessary batch and unit labels based on the actual control recipe executed. This ensures that there is no discrepancy between product and label.
What is an IU?
In pharmacology, the international unit (IU) is a unit of measurement for the amount of a substance, based on measured biological activity or effect.
The unit is used for vitamins, hormones, some medications, vaccines, blood products, and similar biologically active substances.
The precise definition of one IU differs from substance to substance and is established by international agreement for each substance. There is no equivalence among different substances; for instance, one IU of Vitamin E does not contain the same number of milligrams as one IU of Vitamin A.
To define an IU of a substance, the Committee on Biological Standardization of the World Health Organization provides a reference preparation of the substance, arbitrarily sets the number of IUs contained in that preparation, and specifies a biological procedure to compare other preparations of that substance to the reference preparation. The goal in setting the standard is that different preparations with the same biological effect will contain the same number of IUs.
The mass equivalents of 1 IU for selected substances are:
* Vitamin A: 1 IU is the biological equivalent of 0,3 μg retinol, or of 0,6 μg beta-carotene.
* Vitamin C: 1 IU is 50 μg L-ascorbic acid.
* Vitamin D: 1 IU is the biological equivalent of 0,025 μg cholecalciferol/ergocalciferol.
* Vitamin E: 1 IU is the biological equivalent of about 0,667 mg d-alpha-tocopherol (2/3 mg exactly), or of 1 mg of dl-alpha-tocopherol acetate.
Source: www. wikipedia.org
About Advit Animal Nutrition
Advit manufactures a wide assortment of vitamins, minerals and feed additives for the animal feed industry and prepares individual mixes based on each customer’s specific nutritional needs.
The company was formed in 2007 when management of BASF Animal Nutrition in South Africa undertook a management buyout of its Isando premix plant. At the time of the buyout, a supply and technical agreement was signed between the new owners and BASF. The company continues as exclusive distributor of BASF Animal Nutrition products in South Africa and maintains close ties with its German technology partner.
Since the buyout, the plant has been relocated to Sebenza and plant and processes have been progressively upgraded.
Glossary
BCM: ProLoCon’s bar code module
BOM: Bill of materials
COA: Certificate of analysis
GRN: Goods received note
IU: International units
MTM: ProLoCon’s materials transfer module
SOP: Standard operating procedure
UOM: Unit of measure
About the author
Andrew Ashton has electrical, mechanical and business qualifications and has been active in automation and process control since the early 1980s. Since 1991 he has headed up ProLoCon, a company that has developed formulation management systems for the food, pharmaceutical and chemical manufacturing industries and manufacturing solutions involving the integration of various communication technologies and databases. Developed systems address issues around traceability, systems integration, manufacturing efficiency and effectiveness. Andrew is features editor for SA Instrumentation and Control and editor of Motion Control in Southern Africa.

For more information contact Andrew Ashton, ProLoCon, +27 (0)11 465 7861, [email protected], www.prolocon.com

© Technews Publishing (Pty) Ltd | All Rights Reserved